Abstract
INTRODUCTION:
Bispecific chimeric antigen receptor (CAR) T-cell therapies (LV20.19) targeting CD19 and CD20 antigens may improve outcomes in patients with relapsed/refractory (R/R) non-Hodgkin lymphoma (NHL) by limiting CD19-negative relapse. To better understand the impact of manufacturing parameters on CAR T-cell production, we performed single-cell cytokine analysis with the Isoplexis IsoLight instrument on the final LV20.19 CAR T-cell products from a prior trial where IL-2 was used to expand CAR T-cells (NCT03019055) versus LV20.19 CAR products from an ongoing study using IL-7 and IL-15 (IL-7+15) for cell expansion (NCT04186520) in patients with R/R B-cell malignancies.
METHODS:
LV20.19 CAR T-cells were manufactured onsite with the CliniMACS Prodigy device. CAR T-cells were thawed, CD4 & CD8 cells sorted via immunomagnetic separation, the cells stimulated with CD19+ K562 cells, and then loaded onto single-cell Adaptive Immune Isocode chips and read in an IsoLight instrument. The single-cell production of 32 cytokines were measured.
Polyfunctionality (PFA) and polyfunctional strength index (PSI) scores were calculated for each CAR T-cell product using IsoSpeak software. PFA was defined as a T-cell that produced ≥2 cytokines upon stimulation with CD19+ cells. PSI was defined as the percentage of polyfunctional T cells relative to all T cells, multiplied by the sum of the mean fluorescence index of each assayed cytokine from the polyfunctional T cells.
Analysis was limited to those subjects treated at a cell dose of 2.5x106 cells/kg. Variables were analyzed with parametric or non-parametric tests where appropriate and a p-value of <0.05 was considered significant.
RESULTS:
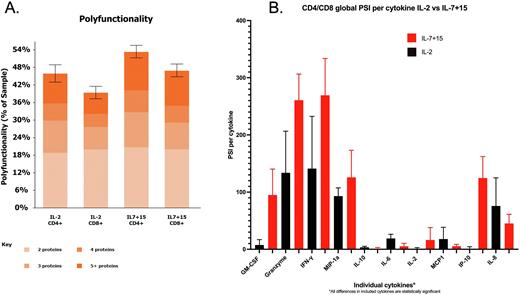
There were 15 patients in the IL-2 trial and 22 patients in the IL-7+15 trial with adequate LV20.19 CAR T-cells for analysis. For cells expanded in IL-7+15 vs IL-2, the global PFA was 53.14% vs 40.12%, p=0.01, CD8 PFA was 51.15% vs 41.24% p=0.01, while the CD4 PFA was 55.05% vs 46.23%, p=0.08, (Figure 1A). Similarly, the global PSI was 1508 vs 956 p=0.0003, CD8 PSI was 1333 vs 865 p=0.0009, and CD4 PSI was 1580 vs 865, p<0.0001. In assessing individual cytokines, the differences seen in both PSI and PFA were largely driven by GM-CSF, granzyme B and IFN-γ (Figure 1B).
We next evaluated the impact of manufacturing time on the cytokine signature of the final LV20.19 products from patients enrolled in the IL-7/IL-15 trial. Products were manufactured for either 8 days (n=13) or 12 days (n=9). The PFA and PSI values between the 2 groups were not statistically different (55.76% vs 51.46% p=0.74 and 1455 vs 1292 p=0.60 respectively).
Next, we evaluated differences in PFA/PSI by NHL disease subtype in the IL-7+15 cohort. We compared 8 patients with mantle cell lymphoma (MCL) vs 11 patients with diffuse large B-cell lymphoma (DLBCL). Again, there were no statistically significant differences between the global PFA and PSI in MCL vs DLBCL; 56.90% vs 51.32% p=0.26 and 1508 vs 1432 p=0.67, respectively. The MCL vs DLBCL CD4 and CD8 PFA and CD4 and CD8 PSI were also not statistically different.
CONCLUSION
Bispecific LV20.19 CAR T-cells expanded in IL7+15 have a higher PFA and PSI than those expanded in IL-2. Whether this change in cytokines improves clinical outcomes for patients is being determined in an ongoing phase 1/2 single-center clinical trial (NCT04186520). Other parameters such as manufacturing time (8 vs 12 days) did not impact PSI or PFA. Interestingly, despite the known relationships of disease biology on the immune system, the PFA and PSI from final products were not impacted by underlying diagnosis (MCL vs DLBCL), demonstrating that functionality of CAR T-cells is independent of the target lymphoma cells.
Disclosures
Fenske:Adaptive Biotechnologies: Consultancy, Speakers Bureau; Beigene: Consultancy; Bristol-Meyers-Squibb: Consultancy, Speakers Bureau; CSL Therapeutics: Consultancy; Karyopharm: Consultancy; Kite (Gilead): Consultancy, Speakers Bureau; MorphoSys: Consultancy, Speakers Bureau; Pharmacyclics (AbbVie): Consultancy; SeaGen: Consultancy, Speakers Bureau; Servier Pharmaceuticals: Consultancy; TG Therapeutics: Consultancy, Speakers Bureau; ADC Therapeutics: Consultancy, Speakers Bureau; Astrazeneca: Speakers Bureau; Sanofi: Speakers Bureau. Johnson:Miltenyi Biotec: Research Funding. Hamadani:Takeda: Research Funding; Novartis: Consultancy; MorphoSys: Consultancy; Sanofi Genzyme: Speakers Bureau; Medical University of Wisconsin: Current Employment; Incyte Corporation: Consultancy; Kite: Consultancy; Genmab: Consultancy; SeaGen: Consultancy; Gamida Cell: Consultancy; Legend Biotech: Consultancy; Kadmon: Consultancy; ADC Therapeutics: Consultancy, Research Funding, Speakers Bureau; Omeros: Consultancy; Abbvie: Consultancy; Spectrum Pharmaceuticals: Research Funding; Astellas Pharma: Research Funding; AstraZeneca: Speakers Bureau; BioGene: Speakers Bureau. Shah:Novartis: Consultancy; Epizyme: Consultancy; Miltenyi Biotec: Consultancy, Research Funding; Lilly Oncology: Consultancy, Honoraria; TG therapeutics: Consultancy; Incyte Corporation: Consultancy, Honoraria, Speakers Bureau; Kite Pharma: Consultancy; Bristol Myers Squibb: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal